FDA’s Failure on Meals Chemical Protection Leaves Individuals at Danger of Serious Illnesses
Tom Neltner, J.D., Chemicals Plan Director and Maricel Maffini, Ph.D., Guide
Update: Food and drug administration published the citizen petition on receipt on 9/23, and is requesting general public comment.
More than 60 several years in the past, Congress enacted laws necessitating the Foodstuff and Drug Administration (Food and drug administration) and the foodstuff industry to consider the cumulative consequences of substances in the food plan that have associated overall health impacts when evaluating the safety of chemical additives. In our 10 years of analyzing Fda and industry steps, we have been increasingly concerned that both of those have disregarded this requirement. To figure it out, we investigated all basic safety determinations contained in Typically Identified as Secure (GRAS) notifications voluntarily submitted by food items companies to Food and drug administration due to the fact the plan began in 1997. We looked at GRAS notices simply because they are publicly available and mainly because Food and drug administration principles explicitly require that meals companies consist of in the recognize an clarification of how they regarded as the prerequisite. If there was an omission, it would be much more conveniently visible.
We observed that in only just one of 877 GRAS notices did a foodstuff manufacturer look at the cumulative influence prerequisite in a significant way. And we discovered no evidence that the agency possibly acknowledged this solitary attempt to abide by the law or experienced objected to the omissions in the 876 other notices. This failure has sizeable repercussions for community wellbeing, specifically for communities who already confront important health and socio-financial disparities, and for little ones, who are uniquely inclined to dietary exposures to a number of substances.
For this explanation, EDF joined with other health, environmental, and shopper teams to file a official petition to demand from customers Fda and food items makers start pursuing the legislation. The petition requests distinct alterations to regulations designed to strengthen the current necessity and make it less complicated to verify compliance. However, provided the lack of transparency in company critiques, results nevertheless largely is dependent on Food and drug administration and the meals marketplace using significantly the mandate and the meals basic safety implications.
The requirement to examine cumulative wellbeing results
When Congress enacted the Food Additives Amendment of 1958, it essential that Fda think about a few issues when evaluating the protection of additives. Between them, the company was directed to think about “the cumulative outcome of these types of additive in the eating plan of male or animals, using into account any chemically or pharmacologically connected substance or substances in such diet plan.”[1]
Six months later, Food and drug administration adopted a rule outlining how the agency and food stuff companies must conduct the expected review for pharmacologically-connected substances. In 1971, it explicitly included the Congressional mandate into its definition of safety. Currently, all basic safety determinations for chemical additives, no matter whether GRAS substances, coloration additives, meals additives, or meals make contact with substances need to take into consideration the cumulative overall health outcome of connected substances.
EDF’s evaluation of GRAS notices
We downloaded 877 generally acknowledged as safe (GRAS) protection selections from FDA’s GRAS observe stock as of March 24, 2020 and analyzed them for conditions “cumulative effect” and “pharmacological.” Figure 1 below summarizes our conclusions.
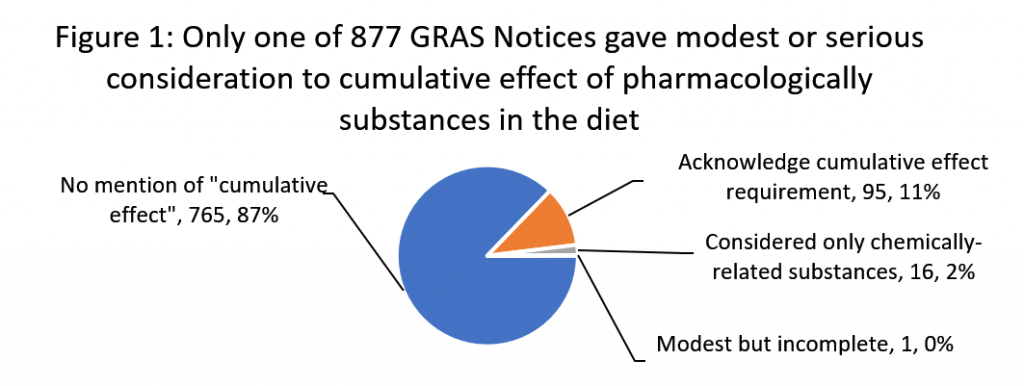
Only a single GRAS observe, GRN 107 for polydextrose, conducted a modest, but incomplete evaluation. It determined 11 pharmacologically-related substances in the diet regime primarily based on their capability to have laxative impact. Having said that, soon after thinking about the average everyday total at which half of the examined subjects knowledge laxation of the substances, the recognize stopped small of establishing a tolerance for the course as essential by Fda rules and only deemed the outcome from polydextrose on your own on the danger of laxation signs.
Food and drug administration and marketplace have neglected their accountability to assess cumulative overall health effects
Though not as fast as adverse drug interactions or foodstuff poisoning, the long-time period, blended impression of food stuff chemicals on our overall health can be sizeable. For case in point, a number of meals additives and contaminants in prevalent foodstuff – together with nitrates, perchlorate, and thiocyanate – all hurt the thyroid’s potential to use iodine to make a hormone vital to brain development. Exposure to these related chemical substances from different foodstuff need to be thought of together, as a course, to minimize the threat for pregnant women of all ages and youthful little ones, as the chemical substances all hurt fetal and infant mind development in the identical way.
Equally field and the Fda all as well typically take into consideration one chemical at a time when evaluating how they affect our overall health – instead than as a class of similar substances, as the agency’s restrictions state. The collective failure by Food and drug administration and the food stuff business to adhere to the law may perhaps effectively have contributed to the spectacular boosts we have viewed in chronic conditions, including weight problems, diabetes, and kidney illness in the US in current a long time. Each and every time we consume extremely processed foods, we are exposed to chemical additives, and – when the chemical substances induce very similar harmful results – that publicity can damage our well being. Regulating chemical compounds with comparable toxicity as a class would lower the over-all publicity to those people substances inside of the course, hence decreasing wellness pitfalls.
The petition outlines the essential techniques for Food and drug administration to consider to make sure that the cumulative influence of a new substance and similar substances previously in the diet plan has been adequately evaluated right before the new additive is authorized in our foodstuff. The petitioners are asking that the company update its regulations, situation direction to field to reveal what is essential to conduct a complete basic safety determination which incorporates accounting for cumulative results and revise its types for market to post notices and petitions.
The petitioners are: EDF American Academy of Pediatrics American Public Wellbeing Association Breast Most cancers Avoidance Partners Middle for Meals Safety Clear Label Project Shopper Federation of The usa Buyer Stories Endocrine Culture Environmental Overall health Tactic Centre Environmental Performing Group and Healthy Babies, Vibrant Futures.
It is time Food and drug administration adopted the regulation and shield American households from the cumulative wellbeing results of chemicals in the food items we try to eat just about every working day.
See the push launch for the petition in this article.
[1] 21 U.S.C. § 348(c)(5)(B)




